In 1960, the first commercial thin film was prepared by phase transformation, thus marking an important milestone in the field of membrane separation technology. After this great invention, gas separation, micro filtration, ultrafiltration and reverse osmosis and other large-scale industrial. At present, the application of membrane separation technology covers almost all industrial fields, such as biotechnology, energy, electronics, environmental and chemical applications. Since the 1980s, several studies have reported the performance characteristics of PVDF membranes. Compared with other commercial polymer materials, polyvinylidene fluoride (PVDF) as a membrane material has attracted much attention. This is because polyvinylidene fluoride material has excellent performance, such as high mechanical strength, good thermal stability, strong chemical resistance, high water resistance, this article is mainly to study and explore the performance of polyvinylidene fluoride material, more in-depth understanding of the characteristics of PVDF.
Polyvinylidene fluoride (PVDF) membrane
In recent years, polyvinylidene fluoride (PVDF) membrane has become one of the most popular membrane materials. Although PVDF membranes are not as hydrophobic as polytetrafluoroethylene (PTFE) and polypropyl (PP), they are more hydrophobic than other materials such as polyimide (PI), polyether sulfone (PE) and polysulfone (PS). Due to the complexity and particularity of solvent selection, the preparation of polypropylene film and polytetrafluoron film by phase conversion method has limitations. Therefore, PVDF is still the best choice of membrane material in the application field, such as the application of membrane distillation and membrane contactor. The reason for this conclusion is that PVDF can be easily dissolved in common organic solvents. Through a series of investigations, it has been shown that the phase transformation method uses a very simple immersion precipitation to prepare porous PVDF membranes. In addition, the excellent thermal stability of PVDF membrane makes it an optimal choice and a popular thin film material in a wide range of industrial fields. Due to its high mechanical strength and excellent chemical resistance, PVDF membrane is a better choice than other membrane materials. Its outstanding performance makes it more suitable for wastewater treatment applications. Furthermore, the PVDF membrane can be purified by a low-level extraction process to produce a pure polymer of its own. This enables it to be widely used in the fields of biomedicine and bioseparation. Different from other crystalline polymers, PVDF itself is highly compatible with other polymers in a variety of mixed solvent components, such as poly (methyl methacrylate) (PMMA), which can help the membrane to perfect its own performance and improve, enhance better performance in the process of membrane preparation. In addition, specific functions can be obtained by further chemical modification, and cross-linking can also be made when exposed to electron beam radiation or gamma radiation.
In addition to PVDF membrane materials, there are some other fluoropolymer membrane materials, such as poly (vinylidene fluoride – hexafluoropropylene) (P (VDF-HFP)), etc. Due to the addition of amorphous phase components of hexafluoropropylene, the content of fluorine is greatly increased, and its hydrophobicity is better than that of PVDF membrane material. Therefore, P (VDF-HFP) membrane material has a more promising application in the application of membrane contactor than PVDF membrane material. P (VDF-HFP) can also be used as a separation material in the field of rechargeable lithium-ion batteries because it contains more amorphous amorphous domain capabilities that can block large amounts of liquid electrolytes.
Thermal stability of PVDF
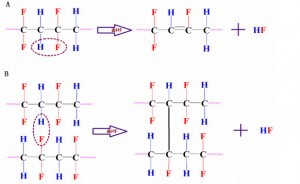
As we all know, PVDF has attracted wide attention in the field of application because of its excellent thermal stability. Fluoropolymers are generally more stable than hydrocarbons. The high electronegativity of the fluorine atoms on the chain and the high dissociation performance of the C-F bond provide the fluoropolymer with ultra-high stability. Although generally stable or inert, degradation of PVDF was observed during high-temperature operations. The thermal degradation of PVDF has been studied in medium, mild and high temperature vacuum. The results showed that the thermal degradation of PVDF at high temperature under vacuum was caused by hydrogen defluorination (or loss of hydrogen fluoride (HF)), which was also the mechanism of thermal degradation. Proximity can lead to a number of chemical reactions, including the formation of carbon-carbon double bonds (C=C) or cross-linking of polymers. FIG. 1 shows a schematic diagram of the possible mechanism of dehydrofluoridation in PVDF. Suggested other possible responses, such as the formation of liberal radicals
The non-uniform thermal degradation of PVDF is the result of crystallization at 160℃. It was observed that PVDF membrane samples showed uneven decolorization at different degradation rates. This is due to the different shapes of the pellets. The degradation process mainly occurs in the crystal region rather than in the amorphous region, whereas this phenomenon does not occur in other polymer degradation processes. The process of degradation is controlled by the disappearance of hydrogen fluoride, which has a small chain break or cross connection. The results of heat treatment show that the composition changes and conformational changes in molecular chains appear in the infrared spectrum due to the existence of a large number of additional absorption bands in the crystallization process, and are long-lasting
 Chemical resistance of PVDF
Chemical resistance of PVDF
PVDF is different from most chemicals in that it is dissolved in partial solvents and has good chemical stability. Some of these solvents include many harsh chemicals, such as halogens and oxidants, inorganic acids, and lipid, aromatic and chlorinated solvents. According to information provided by one of the major suppliers of PVDF, namely Akama Corporation, the chemical stability of PVDF is considered to be the most outstanding among the aforementioned chemicals. However, the good chemical stability of PVDF was seriously damaged for esters, ketones and strong alkali solutions. Therefore, in the application of wastewater treatment or membrane contactor for acid gas absorption, the chemical stability of PVDF membrane is more concerned. The reason is that PVDF membrane is placed in a strong alkaline solution and subjected to alkaline chemical washing, which greatly reduces its stability.
So far, only a few studies on the chemical stability of PVDF membranes have been found relevant to this application. After thermal-alkali treatment, the fluorination color of PVDF films became darker, indicating the formation of C=C bond caused by hydrogen defluorination in the polymer chain. The investigation showed that a chemical attack of sodium hydroxide on the PVDF membrane could accelerate the rate by applying pressure. And when enough pressure is applied, cracks appear in the membrane. They show that sodium hydroxide solution can chemically attack the alpha conformation on PVDF and can observe the fading of PVDF samples immersed in sodium hydroxide solution. At the same time, the reaction between PVDF and sodium hydroxide aqueous solution was studied in the presence or absence of quaternary ammonium salt or phosphorus halide as phase transfer catalyst. The color change of PVDF was observed after the membrane was immersed in a sodium hydroxide solution for a few hours at high temperatures, and this reaction was greatly accelerated with the presence of tetrabutylammonium bromide (TBAB), of which TBAB is a good catalyst for black powders. Fourier transform infrared (FTIR) results revealed the formation of carbon-carbon double and triple bonds, which were attributed to the elimination of hydrogen fluoride molecules. Based on the information described above, the following conclusions can be drawn: In alpha conformation, the sodium hydroxide solution chemically attacks PVDF and
To decolorize its color; With increasing temperature and the presence of catalysts, the chemical attack/reaction of sodium hydroxide on PVDF may be further accelerated. The above factors, such as temperature, pressure, exposure time, frequency of attack cycle, chemical concentration and type of mechanical stress, have a very important influence on the chemistry of PVDF membrane. Relevant research will be of great significance, but it is still very challenging.
The reason why polyvinylidene fluoride film is so concerned and applied is that compared with other polymer material films, it has outstanding properties, such as ultra-high thermal stability, chemical stability and mechanical properties, hydrolysis stability. Now in the membrane application market, PVDF membrane has taken an absolute dominant position, showing ultra-high processing effect. In spite of this, the performance of the membrane will still be affected and some defects will be left during the application process.
1) Due to the high hydrophobicity of PVDF membrane, and the actual application requires the hydrophilic capability of PVDF membrane, the relevant hydrophilic modification of PVDF membrane will be carried out. The treatment process of PVDF membrane may lead to the change of crystallinity or morphology of the membrane, and the near and intercontact grounding may lead to the change of mechanical strength or impact resistance of the membrane.
2) When PVDF membrane is treated at high temperature, it will lead to the defluorination reaction, which greatly reduces the stability of the membrane. Therefore, we must pay attention to the influence of temperature when dealing with PVDF membrane.
3) In addition to the above effects, the chemical resistance of PVDF membrane will be affected by some solvents, such as strong alkali solutions, esters and ketones.
Post time: Dec-02-2022

